What are sparklers made of? Encyclopedia of technologies and techniques
The history of sparklers goes back to ancient India. It was in Bengal, as historians testify, in the 5th-6th centuries. n. e. During religious ceremonies in temples, a fire of unusual brightness flared up on the altars and quickly burned out. At the same time, depending on the wishes of the clergy and the type of ceremony, “it smelled of evil” (probably the composition contained sulfur powder, which when burned forms sulfur dioxide), or “blessed breath” was spread throughout the temple (probably in this case, instead of sulfur, rosin was used in the composition of the sparkler ).
The high efficiency of the action due to the people’s deification of fire and light stimulated the rapid development of the use of fiery compositions and the improvement of their recipes by clergy. By the beginning of the 8th century, the compositions of colored fire were already known - blue, green, yellow. Methods have emerged to lengthen the burning time. To do this, the sparkler composition was filled with hollow, dry plant stems and tubes twisted from dried, wide leaves. These first sparklers not only produced a bright flame, but their burning was also accompanied by a characteristic crackling sound.
In folk life Eastern Slavs Around the same years, “fire fun” took place, which was organized using a club moss. Moss moss or lycopodium, a creeping evergreen herbaceous plant that appears to be moss. Its mature dry spores, when ignited, produce an instant lightning-like flash without smoke.  The flame of a moss is amazing and very fun to watch, especially when thrown at night or in the dark. To make a loud noise, add dried and powdered birch leaves.
The flame of a moss is amazing and very fun to watch, especially when thrown at night or in the dark. To make a loud noise, add dried and powdered birch leaves.
It was in Europe (according to the Spanish researcher Bertrano Luengo - in Valencia) that sparkler torches and indoor compositions first appeared. The next stage on the way to today's Bengal candles, which are familiar to us, was the appearance of sparkling fire recipes in the 6-7 centuries. The effect was achieved by adding iron scale, crushed cast iron, and later magnesium powder to the flaming compositions of the sparkler.
Thus, The sparkler developed in two directions - as fiery and as sparkling. Flame compositions are usually packed in paper sleeves, sparkling ones are applied in several layers to wooden sticks or metal wire.
Russian pyrotechnician Professor Petrov recommended for making flaming sparklers “sleeves made of 3-turn writing paper, 20 mm in cross-section and 35 cm in length. They put 5 cm of clay into the sleeve and then fill it with the fiery composition of a sparkler, lightly tamping it down...” Such a candle burns out along with the sleeve, so it is not possible to hold it in your hands. However, these candles  fixed along the contour of the pattern, due to the even, bright flame they give a wonderful “fiery picture”. If, when making a sleeve, you wind it on wooden stick by 5-7 cm in length, then we will get a flaming Bengal candle that is comfortable to hold in your hands. These products have survived virtually unchanged to this day. The main manufacturers of such candles are China, India, and Japan.
fixed along the contour of the pattern, due to the even, bright flame they give a wonderful “fiery picture”. If, when making a sleeve, you wind it on wooden stick by 5-7 cm in length, then we will get a flaming Bengal candle that is comfortable to hold in your hands. These products have survived virtually unchanged to this day. The main manufacturers of such candles are China, India, and Japan.
 The modern name of these products is triumphal candles. The products are practically smokeless and can be successfully used indoors. At the same time, both individual candles and combined table toys are produced. This is a tabletop figure made up of three or more candles placed in a stand and the upper part pulled together in a tense state. When the product is set on fire, the candles expand and form a multi-beam burning composition.
The modern name of these products is triumphal candles. The products are practically smokeless and can be successfully used indoors. At the same time, both individual candles and combined table toys are produced. This is a tabletop figure made up of three or more candles placed in a stand and the upper part pulled together in a tense state. When the product is set on fire, the candles expand and form a multi-beam burning composition.
Bengal torches are quite popular, especially in Europe. Products are produced different colors(red, green, blue, white, yellow), different sizes(length from 20 cm to 100 cm), for various purposes(outdoor and low-smoke indoor). Bengal torches were an indispensable attribute of all fireworks shows in the 18th and 19th centuries. They were used to set fire to products and in all situations where it was necessary to suddenly illuminate with colored light large area scenes or decorations.
Sparkler- a pyrotechnic product, which is a metal rod coated with a flammable mixture, which, when burned, gives a beautiful sparkling flame. The main advantage of this pyrotechnic product is the ability to be used indoors (outside festive tables), because When fuel burns, no harmful substances are released into the air.
Who invented sparklers

IN ancient india(V – VI centuries AD), on the shores of the Bay of Bengal, religious ceremonies were held in the temples of Bengal. For many years they were carried out using fire. The clergy wanted to involve in the rituals as much as possible more people. To do this, it was necessary to come up with something bright and effective. And so they gave the task to pyrotechnicians to invent such a fire that it would fascinate all people with its flame. It is unknown who exactly invented sparklers, but such a fire appeared at one of the ceremonies. It was of extraordinary brightness and beauty; hundreds of sparks emanated from it, which did not cause any burns. This made a huge impression on people and word of the miracle fire quickly spread throughout the Bay Area. In total, two types of fire were developed: “Evil” and “Blessed”. The first one contained sulfur, which released an unpleasant odor when burned. The second type of fire did not emit an unpleasant odor; apparently, rosin was used instead of sulfur.
In the 8th century Pyrotechnicians invented colored lights: yellow, blue, green. In addition, a way was found to increase the burning time; plant stems and wide leaves twisted into a tube were used for this. Merchants brought sparklers to Europe after trade routes between Europe and India were opened. This type of pyrotechnics quickly gained popularity, and not a single festive event was complete without sparklers.
Modern sparklers do not contain harmful substances. Components: barium nitride, magnesium or aluminum powder, starch or dextrin, oxidized steel filings.
This pyrotechnic product is quite easy to make at home, you just need to acquire the necessary components and mix them in certain proportions.
We present to your attention three compositions described in the book by G.A. Platov “Pyrotechnician. The art of making fireworks." All these recipes are free of sulfur, sodium and potassium salts. Thanks to this, you can use them without fear for your health.
First lineup:
- Barium nitrate 50%
- Blued steel filings 30%
- Dextrin 12 – 14%
- Aluminum powder 6 – 8%
Second composition:
- Barium nitrate 50%
- Cast iron burnished sawdust 30%
- Dextrin 12 – 14%
- Aluminum-magnesium powder (PAM) No. 4 6 – 8%
Third line-up:
- Barium nitrate 50%
- Blued steel filings 30%
- Dextrin 12 – 14%
- Magnesium powder No. 4 6 – 8%
These compositions allow you to make sparklers with your own hands.
We will show an example of manufacturing using a different composition (without using barium nitrate).
Making sparklers
To make 6 - 8 pieces, you will need:
- Cast iron sawdust (medium grain) 5-6 gr.

- Aluminum powder 5 gr.
- Dextrin 2 gr.
- Steel rods (thickness 1 mm.)
Aluminum powder We produce according to a recipe optimally suitable for pyrotechnic products.
To do this, mix:
- Potassium nitrate 50%
- Aluminum powder 35%
- Sulfur 15%
The mixture must be thoroughly ground in a mortar.

Since gunpowder contains sulfur, manufactured sparklers cannot be used indoors.
Dextrin made from starch. Sprinkle starch evenly onto a baking sheet and place it in the oven preheated to 200ºC. We bake it for about an hour and a half, stirring occasionally (make sure that the starch does not melt or roll into lumps). As a result, the powder will turn yellowish-brown in color.

So, all the components are ready and we can start making the sparkler.
We cut the steel wire into pieces 12 - 15 cm long. At one end, we bend these pieces (making a hook). Important! It is necessary to use steel rods; aluminum or copper will not work; they will simply melt when burned.
Pour 5 grams of aluminum powder and 2 grams of dextrin powder into a glass. Mix well, then add 6 grams of metal filings to the mixture (you can add cast iron, they give yellow sparks; aluminum or titanium, they give white sparks). Mix.

Pour the composition into the flask and add a little water or alcohol. Bring the mixture to the consistency of condensed milk.

Now we immerse the prepared steel rods into the substance by 8 - 10 cm. Let the composition adhering to the wire dry. It will take about 15 minutes to dry.

Then apply a second layer and let dry again. Thus, you need to apply 5 layers. During drying, do not forget to cover the flask so that the liquid does not evaporate from the composition.
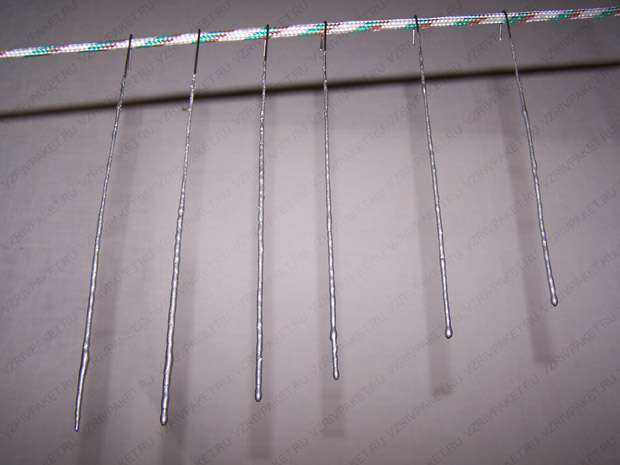
This is what DIY sparklers will look like.

It's time for testing.
As you can see, homemade sparklers (specifically these ones) produce few sparks. The reason for this is the fact that during manufacturing only 3 grams of cast iron filings were added to the composition. But if you strictly follow the instructions described above and add 6 grams of sawdust to the composition, then there will be much more sparks (as in real lights).
Based on materials from the site: vzrivpaket.com
Sparklers are an indispensable attribute of any happy holiday- New Year, birthday (and not only children’s), wedding. They cost pennies, are sold everywhere, and are absolutely safe for lighting indoors. However, people still continue to be interested in how to make sparklers at home: creating colorful joy with their children is both interesting and increases the anticipation of the holiday. Moreover, the process itself is simple, and necessary substances quite accessible.
Composition recipes
Craftsmen have come up with several ways to do it yourself. Depending on what is easier for you to get, you can choose any of the recipes. In one group we include compounds that must include 50% of total mass barium nitrate and 12-14 percent dextrin. Additional ingredients can be selected from the following list:
- From 6 to 8% fine aluminum powder plus 30% sawdust - always steel and burnished.
- The same amount of similar sawdust is added to the base, and the powder is replaced with PAM No. 4 - this is short for aluminum-magnesium powder.
All options are good because they do not contain potassium/sodium salts or sulfur, so the candles will not produce smoke, as well as various kinds of toxins. Result: lights can be used in the home without fear of poisoning.
Another composition has a radically different recipe. For 6-8 lights, take aluminum gunpowder weighing 5 grams, dextrin - 2 grams and sawdust, this time cast iron, which will take from 5 to 6 g. These lights will have to be used only in the fresh air. 
Obtaining the necessary ingredients
It is not always possible to buy some of the necessary substances. So before you make a sparkler at home, you may have to start preparing the components. The simplest situation is with dextrin: it is formed when ordinary starch is heated in the oven. The temperature is set to 195 Celsius, processing is carried out until the original substance acquires a brownish tint.
If you decide on a recipe with aluminum gunpowder, you will have to do it outdoors. Aluminum powder and sulfur are ground in a mortar in proportions of 30:45:25. 
Important: wire
It is worth paying attention to the base by which you will hold your “fireworks”. There are also several subtleties here. First, the length: cut the wire into pieces of at least 14 cm. This way you will protect your hand from sparks or hot metal. Secondly, thickness. Take wire with a cross-section of 2-3 mm. A thinner one may burn out and break. Thirdly, the material. Neither copper nor aluminum are suitable - the combustion temperature of the mixtures is high, and they will simply melt. Look and don’t forget, before making a sparkler at home, bend one end into a hook so that you can hang it to dry. If the bend bothers you, you can straighten it later or bite it off.
How to make a sparkler with your own hands: instructions
In addition to all the chemicals and rods, you will need a container in which to prepare the composition. First, aluminum gunpowder or barium salt is poured into it, then dextrin, and only after mixing - the remaining components of the selected mixture. When the dry powder is mixed until approximately homogeneous, a little solvent is poured into it (water is possible, but alcohol is better). The consistency should resemble boiled condensed milk. A piece of wire is lowered into the composition, leaving 5-7 cm for the “handle”. The future sparkler is hung to dry for a quarter of an hour, after which the manipulations are repeated twice more. Three layers will dry for half an hour, and then two more are applied in the manner already described. Final drying will take half a day, and you can set it on fire. 
Another way
Often people use a slightly different technology on how to make sparklers at home. First, a strong paste is prepared from starch and tap water. Then an incendiary mixture is ground, which includes fine-grained iron filings, magnesium powder (alternatively aluminum), kitchen salt and Bertoletova. The latter must be handled carefully and wetted a little before pouring. The mixture is kneaded in brewed starch until smooth. The rods are dipped into the finished product in the same way with time intervals between applying layers.
If you read books about misfits, then there main character loves to amaze dark audiences with ingenious crafts.
A classic sparkler is quite suitable for this.
At the same time, sparklers as such are very ancient invention. But how much ancient fire exactly as we know it?
The sparkler is so named because it was invented (surprise!) on the shores of the Bay of Bengal in India. And was used for signaling and special effects in temples. Well, at least this is all according to legend, because it all happened in the 5th - 6th century.
You can find many compositions of sparklers, there are definitely more than a dozen of them.
The main thing is that they have several properties. Firstly close relative sparklers are thermite mixtures that burn at temperatures above 2400°C and, as a result, have a very bright flame. Secondly, the composition of a sparkler includes not only fuel (in this case metal) but also an oxidizer, so the composition of a sparkler is somewhat similar to the composition of gunpowder.
It was in this form that sparklers came to Europe. However, at first it was just a bright fire, and then they began to add crushed iron scale or cast iron (iron or steel) filings to it. They are the ones who produce these bright yellow stars that scatter in all directions. And they are also responsible for the characteristic sound of a sparkler burning. This was already invented in Europe, somewhere in the 6th - 7th century (in Valencia).
I haven't found an ancient sparkler recipe, but let's see what's included now:
1. A metal that oxidizes. Aluminum and magnesium produce a clean white flame. Titanium gives a particularly bright color. Nowadays an alloy of aluminum and magnesium with additions of nickel and tin is also used.
2. An additional fuel that controls the temperature and rate of combustion. These are sulfur and coal.
3. Oxidizing agents. Nitrates of barium, strontium, potassium. Potassium perchlorate KClO 4 (not to be confused with Berthollet salt, which is KClO 3, this one will be cooler).
4. Additional pyrotechnic dyes for colored flames. Typically barium and strontium chlorides or copper.
5. The combustible filler is dextrin or nitrocellulose.
You can dig up a mountain of recipes, here are the classic ones:
Composition No. 1
Barium nitrate…………………………..50%
Dextrin…………………………………12-14%
Aluminum powder………………..6-8%
Burnished steel filings…..30%
Composition No. 2
Barium nitrate……………………………………………………..50%
Dextrin……………………………………………………………..12-14%
Aluminum-Magnesium powder No. 4….6-8%
Burnished cast iron sawdust………………….30%
Composition No. 3
Barium nitrate……………………………50%
Dextrin………………………………….12-14%
Magnesium powder No. 4…………..6-8%
Burnished steel filings………30%
By by and large, I consider the invention of something like this to be a failed idea. No matter how much the author of the book would like to invent pyrotechnic effects.
The fact is that since ancient times, humanity has had fun - throwing whatever comes to hand into the fire and seeing what comes out. This is how the first ceramics were produced and the first metal was smelted.
Unless these will be very ancient times, but then what kind of metal should be burned? And what oxidizing agent? Wouldn't it be too much effort for such a narrow niche?
Burning a sparkler at a temperature of 1100° C
Sparklers- a mixture of substances that, when burned, gives a bright and sparkling white or colored fire, was invented by the ancient pyrotechnics of Bengal - a part of India located along the Bay of Bengal. This is where the name “Bengal fire” comes from. Bengal lights, or sparklers, from India spread throughout the world.
Making sparklers
Store-bought sparklers consist of twisted wire coated with a flammable mixture and typically produce a white flame. To prepare colored homemade sparklers, first mix starch with water and brew a thick paste.
Then grind in a mortar a mixture of iron filings, aluminum or magnesium powder, flame-coloring salt and wet “Berthollet salt” - potassium chlorate KClO3 (Caution! Dry potassium chlorate, when ground, can ignite metal powders!)
The mixture obtained by grinding is added to the starch paste and mixed thoroughly. The thick mass is transferred to a test tube or a tall glass, pre-prepared iron wires about 1 mm thick are alternately dipped into it to a depth of 8-10 cm, taken out and allowed to drain off the excess mass, and then hung on a rope by a hook bent at the other end of the wire.
After drying, the wires are again dipped into the liquid mass and dried again. These operations are repeated 3-5 times until the layer of mass on the wire reaches 5-6 mm in diameter, after which the sparklers are dried completely.
Green sparkler is prepared by mixing without grinding 5 g of wet barium nitrate Ba(NO3)2 with 1 g of aluminum or magnesium powder, then adding 3 g of iron filings. Another recipe for a green sparkler includes 3.5g boric acid B(OH)3, 6.5g wet potassium chlorate, 2g iron filings and 1g aluminum powder.
A red sparkler produces a mixture of 4.5 g wet strontium nitrate Sr(NO3)2, 5.5 g potassium chlorate, 3 g iron filings and 1 g aluminum or magnesium powder.
A yellow sparkler will delight your eyes if it is prepared from 3 g sodium oxalate Na2C2O4, 5 g wet potassium chlorate, 3 g iron filings and 1 g aluminum or magnesium powder.
Reactions
Colored fire when burning sparkler mixtures is obtained due to the presence of substances containing cations of barium, strontium, sodium or boron atoms, capable of emitting light of a certain wavelength in the visible region of the spectrum when entering the flame. Iron Fe, aluminum Al and magnesium Mg in the form of powders or fine sawdust, when burned, produce spectacular sparks. In this case, iron(III) oxide Fe 2 O 3 and partly Fe 3 O4, as well as Al 2 O 3 and MgO are formed.
Na 2 C 2 O 4 = Na 2 CO 3 + CO
and boric acid B(OH) 3, releasing water, turns into boron oxide:
2B(OH) 3 = B 2 O 3 + 3H 2 O By the way: what are “oxalates”?
Oxalates are salts of oxalic acid H 2 C 2 O 4. 2H 2 O, a colorless crystalline substance. Alkali metal and ammonium oxalates are colorless crystalline substances, highly soluble in water; the remaining oxalates are slightly soluble.
Strong acids in their concentrated aqueous solutions decompose oxalates into salts of these acids, releasing carbon monoxide and carbon dioxide. For example, sodium oxalate Na2C2O4 under the action of concentrated sulfuric acid is converted into sodium sulfate, releasing CO and CO2:
Na 2 C 2 O 4 + H 2 SO 4 = Na 2 SO 4 + CO + CO 2 + H 2 O
Oxalic acid is dibasic and forms two series of salts: medium ones, for example, potassium oxalate monohydrate K 2 C 2 O 4. H 2 O, and acidic - hydroxalates, for example, potassium hydroxalate monohydrate KHC 2 O 4. H 2 O. When heated, almost all oxalates decompose into metal carbonates and carbon monoxide CO. Thus, calcium oxalate CaC 2 O 4 turns into calcium carbonate and carbon monoxide: - a pyrotechnic composition, the combustion of which is accompanied by the scattering of sparkling sparks. Typically, sparkler candles are applied to pieces of metal wire. The name comes from the signaling method first used in Bengal (India) using... ... Encyclopedic Dictionary
sparkler- bengališkoji ugnis statusas T sritis chemija apibrėžtis Pirotechninis mišinys, susidedantis iš sieros, cukraus, KNO₃ ir Ba ar Sr druskų. atitikmenys: engl. bengal lights; sparklers rus. sparkler... Chemijos terminų aiškinamasis žodynas
A pyrotechnic composition containing barium nitrate (oxidizer), powdered aluminum or magnesium (fuel), dextrin or starch (cementant), and oxidized iron or steel filings. The composition is applied to pieces of iron... ... Great Soviet Encyclopedia
So-called in pyrotechnics, a composition that emits bright white or colored light when burned. It got its name from the method of signaling used for the first time in India using light, obtained by burning a mixture of 16 parts in bamboo tubes... ... Encyclopedic Dictionary F.A. Brockhaus and I.A. Efron
- (from the name of the historical region in India, Bengal) pyrotechnic. composition containing barium nitrate (oxidizing agent), powdered aluminum or magnesium, iron. or steel filings (fuel) and dextrin or starch (cementant). When setting fire to B. o. slowly… … Big Encyclopedic Polytechnic Dictionary
sparkler- A pyrotechnic composition that burns with a bright white or colored flame and scatters sparks... Dictionary of many expressions
Fire, m. 1. only units. Hot glowing gases released from burning objects; flame. Strong o. Blow up about. (see bloat). Build a fire (see make a fire). Warm up what n. on fire. || Same as a fire source. Fire insurance. 2. pl.… … Dictionary Ushakova
BENGAL, Bengal, Bengal. adj. to Bengal (province of India). Bengal tiger. ❖ Bengal fire is a pyrotechnic composition for illumination, burning with colored fire. Ushakov's explanatory dictionary. D.N. Ushakov. 1935 1940 ... Ushakov's Explanatory Dictionary








 About the company Foreign language courses at Moscow State University
About the company Foreign language courses at Moscow State University Which city and why became the main one in Ancient Mesopotamia?
Which city and why became the main one in Ancient Mesopotamia? Why Bukhsoft Online is better than a regular accounting program!
Why Bukhsoft Online is better than a regular accounting program! Which year is a leap year and how to calculate it
Which year is a leap year and how to calculate it