Chemistry tutor manual. Alkali metals and their compounds Potassium iodide solution was treated with excess chlorine water
Tasks C 2 (2013)
Reactions confirming the relationship between various classes of inorganic substances
Copper(II) oxide was heated in a stream of carbon monoxide. The resulting substance was burned in a chlorine atmosphere. The reaction product was dissolved in water. The resulting solution was divided into two parts. A solution of potassium iodide was added to one part, and a solution of silver nitrate was added to the second. In both cases, the formation of a precipitate was observed. Write equations for the four reactions described.
Copper nitrate was calcined, and the resulting solid was dissolved in dilute sulfuric acid. The solution of the resulting salt was subjected to electrolysis. The substance released at the cathode was dissolved in concentrated nitric acid. Dissolution proceeded with the release of brown gas. Write equations for the four reactions described.
The iron was burned in a chlorine atmosphere. The resulting substance was treated with an excess of sodium hydroxide solution. A brown precipitate formed, which was filtered and calcined. The residue after calcination was dissolved in hydroiodic acid. Write equations for the four reactions described.
Aluminum metal powder was mixed with solid iodine and a few drops of water were added. A solution of sodium hydroxide was added to the resulting salt until a precipitate formed. The resulting precipitate was dissolved in hydrochloric acid. Upon subsequent addition of sodium carbonate solution, precipitation was again observed. Write equations for the four reactions described.
As a result of incomplete combustion of coal, a gas was obtained, in the current of which iron(III) oxide was heated. The resulting substance was dissolved in hot concentrated sulfuric acid. The resulting salt solution was subjected to electrolysis. Write equations for the four reactions described.
A certain amount of zinc sulfide was divided into two parts. One of them was treated with nitric acid, and the other was fired in air. When the released gases interacted, a simple substance was formed. This substance was heated with concentrated nitric acid, and a brown gas was released. Write equations for the four reactions described.
Sulfur was fused with iron. The reaction product was dissolved in water. The gas released was burned in excess oxygen. The combustion products were absorbed by an aqueous solution of iron(III) sulfate. Write equations for the four reactions described.
The iron was burned in chlorine. The resulting salt was added to the sodium carbonate solution, and a brown precipitate formed. This precipitate was filtered and calcined. The resulting substance was dissolved in hydroiodic acid. Write equations for the four reactions described.
A solution of potassium iodide was treated with an excess of chlorine water, and first the formation of a precipitate was observed, and then its complete dissolution. The resulting iodine-containing acid was isolated from the solution, dried and carefully heated. The resulting oxide reacted with carbon monoxide. Write down the equations for the reactions described.
Chromium(III) sulfide powder was dissolved in sulfuric acid. At the same time, gas was released and a colored solution was formed. An excess of ammonia solution was added to the resulting solution, and the gas was passed through lead nitrate. The resulting black precipitate turned white after treatment with hydrogen peroxide. Write down the equations for the reactions described.
Aluminum powder was heated with sulfur powder, and the resulting substance was treated with water. The resulting precipitate was treated with an excess of a concentrated solution of potassium hydroxide until it was completely dissolved. A solution of aluminum chloride was added to the resulting solution and the formation of a white precipitate was again observed. Write down the equations for the reactions described.
Potassium nitrate was heated with powdered lead until the reaction stopped. The mixture of products was treated with water, and then the resulting solution was filtered. The filtrate was acidified with sulfuric acid and treated with potassium iodide. The isolated simple substance was heated with concentrated nitric acid. Red phosphorus was burned in the atmosphere of the resulting brown gas. Write down the equations for the reactions described.
Copper was dissolved in dilute nitric acid. An excess of ammonia solution was added to the resulting solution, observing first the formation of a precipitate, and then its complete dissolution with the formation of a dark blue solution. The resulting solution was treated with sulfuric acid until the characteristic blue color of copper salts appeared. Write down the equations for the reactions described.
Magnesium was dissolved in dilute nitric acid, and no gas evolution was observed. The resulting solution was treated with an excess of potassium hydroxide solution while heating. The gas released was burned in oxygen. Write down the equations for the reactions described.
A mixture of potassium nitrite and ammonium chloride powders was dissolved in water and the solution was gently heated. The released gas reacted with magnesium. The reaction product was added to an excess of hydrochloric acid solution, and no gas evolution was observed. The resulting magnesium salt in solution was treated with sodium carbonate. Write down the equations for the reactions described.
Aluminum oxide was fused with sodium hydroxide. The reaction product was added to a solution of ammonium chloride. The released gas with a pungent odor is absorbed by sulfuric acid. The resulting medium salt was calcined. Write down the equations for the reactions described.
Chlorine reacted with a hot solution of potassium hydroxide. As the solution cooled, crystals of Berthollet salt precipitated. The resulting crystals were added to a solution of hydrochloric acid. The resulting simple substance reacted with metallic iron. The reaction product was heated with a new portion of iron. Write down the equations for the reactions described.
Copper was dissolved in concentrated nitric acid. An excess of ammonia solution was added to the resulting solution, observing first the formation of a precipitate, and then its complete dissolution. The resulting solution was treated with excess hydrochloric acid. Write down the equations for the reactions described.
Iron was dissolved in hot concentrated sulfuric acid. The resulting salt was treated with an excess of sodium hydroxide solution. The brown precipitate that formed was filtered and calcined. The resulting substance was fused with iron. Write equations for the four reactions described.
|
1)CuO + CO=Cu+CO 2 2) Cu+Cl 2 = CuCl 2 3) 2CuCl 2 +2KI=2CuCl↓ +I 2 +2KCl 4) CuCl 2 +2AgNO 3 =2AgCl↓+Cu(NO 3) 2 |
1)Cu(NO 3) 2 2CuO+4NO 2 +O 2 2) CuO+2H 2 SO 4 =CuSO 4 +SO 2 +2H 2 O 3)CuSO 4 +H 2 O=Cu↓+H 2 SO 4 +O 2 (elect 4)Cu+4HNO 3 =Cu(NO 3) 2 +2NO 2 +2H 2 O |
1) 2Fe + 3Cl 2 = 2FeCl 3 2)FeCl 3 + 3NaOH = Fe(OH) 3 ↓+3NaCl 4)Fe 2 O 3 + 6HI = 2FeI 2 + I 2 + 3H 2 O |
|
1) 2Al+3I 2 = 2AlI 3 2) AlI 3 +3NaOH= Al(OH) 3 +3NaI 3)Al(OH) 3 + 3HCl= AlCl 3 + 3H 2 O 4)2AlCl 3 +3Na 2 CO 3 +3H 2 O=2Al(OH) 3 +3CO 2 +6NaCl |
2) Fe 2 O 3 +CO=Fe+CO 2 3)2Fe+6H 2 SO 4 =Fe 2 (SO 4) 3 +3SO 2 +6H 2 O 4)Fe 2 (SO 4) 3 +4H 2 O=2Fe+H 2 +3H 2 SO 4 +O 2 (electrolysis) |
1) ZnS+2HNO 3 =Zn(NO 3) 2 +H 2 S 2)2ZnS +3O 2 =2ZnO +2SO 2 3)2H 2 S+SO 2 =3S↓+2H 2 O 4)S+6HNO 3 =H 2 SO 4 +6NO 2 +2H 2 O |
|
2) FeS + 2H 2 O=Fe(OH) 2 +H 2 S 3)2H 2 S+3O 2 2SO 2 +2H 2 O 4)Fe 2 (SO 4) 3 +SO 2 +2H 2 O=2FeSO 4 + 2H 2 SO 4 |
1) 2Fe + 3Cl 2 = 2FeCl 3 2)2FeCl 3 +3Na 2 CO 3 =2Fe(OH) 3 +6NaCl+3CO 2 3) 2Fe(OH) 3 Fe 2 O 3 + 3H 2 O 4) Fe 2 O 3 + 6HI = 2FeI 2 + I 2 + 3H 2 O |
1)2KI+Cl 2 =2KCl+I 2 2)I 2 +5Cl 2 +6H 2 O=10HCl+2HIO 3 3)2HIO 3 I 2 O 5 + H 2 O 4) I 2 O 5 +5CO = I 2 +5CO 2 |
|
1)Cr 2 S 3 +3H 2 SO 4 =Cr 2 (SO 4) 3 +3H 2 S 2)Cr 2 (SO 4) 4 +6NH 3 +6H 2 O=2Cr(OH) 3 ↓+3(NH 4) 2 SO 4 3)H 2 S+Pb(NO 3) 2 =PbS↓+2HNO 3 4)PbS+4H 2 O 2 =PbSO 4 +4H 2 O |
1)2Al+3S Al 2 S 3 2)Al 2 S 3 +6H 2 O=2Al(OH) 3 ↓+3H 2 S 3)Al(OH) 3 +KOH=K 4)3K+AlCl 3 =3KCl+Al(OH) 3 ↓ |
1)KNO 3 +Pb KNO 2 +PbO 2)2KNO 2 +2H 2 SO 4 +2KI=2K 2 SO 4 + 2NO+I 2 +2H 2 O 3)I 2 +10HNO 3 2HIO 3 +10NO 2 +4H 2 O 4)10NO 2 +P=2P 2 O 5 +10NO |
|
1)3Cu+8HNO 3 =3Cu(NO 3) 2 +2NO+4H 2 O 4)(OH) 2 +3H 2 SO 4 = CuSO 4 +2(NH 4) 2 SO 4 + 2H 2 O |
1)4Mg+10HNO3 = 4Mg(NO3)2 +NH4NO3 + 3H2O 2) Mg(NO 3) 2 +2KOH=Mg(OH) 2 ↓+2KNO 3 3)NH 4 NO 3 +KOHKNO 3 +NH 3 +H 2 O 4)4NH 3 +3O 2 =2N 2 +6H 2 O |
1)KNO 2 +NH 4 Cl KCl+N 2 +2H 2 O 2) 3Mg+N 2 =Mg 3 N 2 3)Mg 3 N 2 +8HCl=3MgCl 2 +2NH 4 Cl 4)2MgCl 2 +2Na 2 CO 3 +H 2 O= (MgOH) 2 CO 3 ↓+ CO 2 +4NaCl |
|
1)Al 2 O 3 +2NaOH 2NaAlO 2 +H 2 O 2)NaAlO 2 +NH 4 Cl+H 2 O=NaCl+ Al(OH) 3 ↓+NH 3 3)2NH 3 +H 2 SO 4 =(NH 4) 2 SO 4 4)(NH 4) 2 SO 4 NH 3 +NH 4 HSO 4 |
1)3Cl 2 +6KOH6KCl+KClO 3 +3H 2 O 2)6HCl+KClO 3 =KCl+3Cl 2 +3H 2 O 3)2Fe+3Cl 2 =2FeCl 3 4)2FeCl 3 +Fe3FeCl 2 |
1)3Cu+4HNO 3 =3Cu(NO 3) 2 +2NO 2 +4H 2 O 2)Cu(NO 3) 2 +2NH 3 H 2 O=Cu(OH) 2 + 2NH 4 NO 3 3)Cu(OH) 2 +4NH 3 H 2 O =(OH) 2 + 4H 2 O 4)(OH) 2 +6HCl= CuCl 2 +4NH 4 Cl + 2H 2 O |
|
19 Document C 2 Reactions, confirming relationship various classes inorganic substances Given aqueous solutions... Write equations for the four possible reactions. Dans substances: hydrobromic acid, ... precipitates during reactions substance yellow color burned on... Summary report of the chairmen of subject commissions of the Astrakhan region on academic subjects of the state final certification for educational programs of secondary general educationReport4 21.9 40.6 C2 Reactions, confirming relationship various classes inorganic substances 54.1 23.9 9.9 7.7 4.3 C3 Reactions, confirming relationship organic compounds 56.8 10 ... Calendar-thematic planning of classes in preparation for the Unified State Exam in chemistry in the 2013–2014 academic year SubjectCalendar and thematic planning25) 27.03.2014 Reactions, confirming relationship various classes inorganic substances. Solution of exercises C-2...simple substances. Chemical properties of complex substances. Relationship various classes inorganic substances. Reactions ion exchange... Calendar-thematic lesson plan for preparing for the Unified State Exam in Chemistry for school graduates in Barnaul and the Altai Territory in 2015. Lesson numberCalendar-thematic plan... (using the example of aluminum and zinc compounds). Relationship inorganic substances. Reactions, confirming relationship various classes inorganic substances(37 (C2)). February 28, 2015... |
Chemistry tutor
LESSON 10
10th grade(first year of study)
Continuation. For the beginning, see No. 22/2005; 1, 2, 3, 5, 6, 8, 9, 11/2006
Redox reactions
Plan
1. Oxidation-reduction reactions (ORR), degree of oxidation.
2. Oxidation process, the most important reducing agents.
3. The reduction process, the most important oxidizing agents.
4. Redox duality.
5. Main types of ORR (intermolecular, intramolecular, disproportionation).
6. ORR value.
7. Methods for compiling ORR equations (electronic and electron-ion balance).
All chemical reactions based on changes in the oxidation states of the atoms involved can be divided into two types: ORR (those that occur with a change in oxidation states) and non-ORR.
Oxidation state– the conditional charge of an atom in a molecule, calculated based on the assumption that only ionic bonds exist in the molecule.
Rules for determining the degree of oxidation
The oxidation state of atoms of simple substances is zero.
The sum of the oxidation states of atoms in a complex substance (in a molecule) is zero.
The oxidation state of alkali metal atoms is +1.
The oxidation state of alkaline earth metal atoms is +2.
The oxidation state of boron and aluminum atoms is +3.
The oxidation state of hydrogen atoms is +1 (in hydrides of alkali and alkaline earth metals –1).
The oxidation state of oxygen atoms is –2 (in peroxides –1).
Any ORR is a set of processes of electron donation and addition.
The process of giving up electrons is called oxidation. Particles (atoms, molecules or ions) that donate electrons are called restorers. As a result of oxidation, the oxidation state of the reducing agent increases. Reducing agents can be particles in lower or intermediate oxidation states. The most important reducing agents are: all metals in the form of simple substances, especially active ones; C, CO, NH 3, PH 3, CH 4, SiH 4, H 2 S and sulfides, hydrogen halides and metal halides, metal hydrides, metal nitrides and phosphides.
The process of adding electrons is called restoration. Particles that accept electrons are called oxidizing agents. As a result of reduction, the oxidation state of the oxidizing agent decreases. Oxidizing agents can be particles in higher or intermediate oxidation states. The most important oxidizing agents: simple non-metal substances with high electronegativity (F 2, Cl 2, O 2), potassium permanganate, chromates and dichromates, nitric acid and nitrates, concentrated sulfuric acid, perchloric acid and perchlorates.
There are three types of redox reactions.
Intermolecular OVR - an oxidizing agent and a reducing agent are included in various substances, for example:

Intramolecular OVR – an oxidizing agent and a reducing agent are part of one substance. These can be different elements, for example:

or one chemical element in different oxidation states, for example:

Disproportionation (auto-oxidation-self-healing)– the oxidizing agent and the reducing agent are the same element, which is in an intermediate oxidation state, for example:

ORRs are of great importance, since most reactions occurring in nature belong to this type (photosynthesis process, combustion). In addition, ORRs are actively used by humans in their practical activities (reduction of metals, synthesis of ammonia):

To compile ORR equations, you can use the electronic balance (electronic circuits) method or the electron-ion balance method.
Electronic balance method:
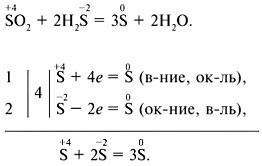
Electron-ion balance method:

Test on the topic “Oxidation-reduction reactions”
1. Potassium dichromate was treated with sulfur dioxide in a sulfuric acid solution, and then with an aqueous solution of potassium sulfide. The final substance X is:
a) potassium chromate; b) chromium(III) oxide;
c) chromium(III) hydroxide; d) chromium(III) sulfide.
2. Which reaction product between potassium permanganate and hydrobromic acid can react with hydrogen sulfide?
a) Bromine; b) manganese(II) bromide;
c) manganese dioxide; d) potassium hydroxide.
3. The oxidation of iron(II) iodide with nitric acid produces iodine and nitrogen monoxide. What is the ratio of the coefficient of the oxidizing agent to the coefficient of the reducing agent in the equation of this reaction?
a) 4: 1; b) 8: 3; c) 1:1; d) 2:3.
4. The oxidation state of the carbon atom in the bicarbonate ion is equal to:
a) +2; b) –2; c) +4; d) +5.
5. Potassium permanganate in a neutral environment is reduced to:
a) manganese; b) manganese(II) oxide;
c) manganese(IV) oxide; d) potassium manganate.
6. The sum of the coefficients in the equation for the reaction of manganese dioxide with concentrated hydrochloric acid is equal to:
a) 14; b) 10; c) 6; d) 9.
7. Of the listed compounds, only the following exhibit oxidizing ability:
a) sulfuric acid; b) sulfurous acid;
c) hydrosulfide acid; d) potassium sulfate.
8. Of the listed compounds, redox duality is exhibited by:
a) hydrogen peroxide; b) sodium peroxide;
c) sodium sulfite; d) sodium sulfide.
9. Of the following types of reactions, redox reactions are:
a) neutralization; b) restoration;
c) disproportionation; d) exchange.
10. The oxidation state of a carbon atom does not numerically coincide with its valency in the substance:
a) carbon tetrachloride; b) ethane;
c) calcium carbide; d) carbon monoxide.
Key to the test
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| V | A | A | V | V | G | a, d | a, b, c | b, c | b, c |
Exercises on redox reactions
(electronic and electron-ion balance)
Task 1. Create OVR equations using the electronic balance method, determine the type of OVR.
1. Zinc + potassium dichromate + sulfuric acid = zinc sulfate + chromium(III) sulfate + potassium sulfate + water.
Electronic balance:

2. Tin(II) sulfate + potassium permanganate + sulfuric acid = tin(IV) sulfate + manganese sulfate + potassium sulfate + water.
3. Sodium iodide + potassium permanganate + potassium hydroxide = iodine + potassium manganate + sodium hydroxide.
4. Sulfur + potassium chlorate + water = chlorine + potassium sulfate + sulfuric acid.
5. Potassium iodide + potassium permanganate + sulfuric acid = manganese(II) sulfate + iodine + potassium sulfate + water.
6. Iron(II) sulfate + potassium dichromate + sulfuric acid = iron(III) sulfate + chromium(III) sulfate + potassium sulfate + water.
7. Ammonium nitrate = nitric oxide (I) + water.
8. Phosphorus + nitric acid = phosphoric acid + nitric oxide (IV) + water.
9. Nitrous acid = nitric acid + nitric oxide (II) + water.
10. Potassium chlorate + hydrochloric acid = chlorine + potassium chloride + water.
11. Ammonium dichromate = nitrogen + chromium(III) oxide + water.
12. Potassium hydroxide + chlorine = potassium chloride + potassium chlorate + water.
13. Sulfur(IV) oxide + bromine + water = sulfuric acid + hydrobromic acid.
14. Sulfur(IV) oxide + hydrogen sulfide = sulfur + water.
15. Sodium sulfite = sodium sulfide + sodium sulfate.
16. Potassium permanganate + hydrochloric acid = manganese(II) chloride + chlorine + potassium chloride + water.
17. Acetylene + oxygen = carbon dioxide + water.
18. Potassium nitrite + potassium permanganate + sulfuric acid = potassium nitrate + manganese(II) sulfate + potassium sulfate + water.
19. Silicon + potassium hydroxide + water = potassium silicate + hydrogen.
20. Platinum + nitric acid + hydrochloric acid = platinum(IV) chloride + nitric oxide + water.
21. Arsenic sulfide + nitric acid = arsenic acid + sulfur dioxide + nitrogen dioxide + water.
22. Potassium permanganate = potassium manganate + manganese(IV) oxide + oxygen.
23.
Copper(I) sulfide + oxygen + calcium carbonate = copper(II) oxide + calcium sulfite +
+ carbon dioxide.
24.
Iron(II) chloride + potassium permanganate + hydrochloric acid = iron(III) chloride + chlorine +
+ manganese(II) chloride + potassium chloride + water.
25. Iron(II) sulfite + potassium permanganate + sulfuric acid = iron(III) sulfate + manganese(II) sulfate + potassium sulfate + water.
Answers to exercises in task 1


When using the half-reaction method (electron-ion balance), it should be borne in mind that in aqueous solutions the binding of excess oxygen and the addition of oxygen by a reducing agent occurs differently in acidic, neutral and alkaline media. In acidic solutions, excess oxygen is bound by protons to form water molecules, and in neutral and alkaline solutions by water molecules to form hydroxide ions. The addition of oxygen by a reducing agent is carried out in acidic and neutral environments due to water molecules with the formation of hydrogen ions, and in an alkaline environment - due to hydroxide ions with the formation of water molecules.
Neutral environment:
Alkaline environment:
oxidizing agent + H 2 O = ... + OH – ,
reducing agent + OH – = ... + H 2 O.
Acidic environment:
oxidizing agent + H + = ... + H 2 O,
reducing agent + H 2 O = ... + H + .
Task 2. Using the electron-ion balance method, compose equations for redox reactions occurring in a certain environment.
1. Sodium sulfite + potassium permanganate + water = .......................
2. Iron(II) hydroxide + oxygen + water = .....................................
3. Sodium bromide + potassium permanganate + water = ..........................
4. Hydrogen sulfide + bromine + water = sulfuric acid + .......................
5. Silver(I) nitrate + phosphine + water = silver + phosphoric acid + ..................................
IN ALKALINE ENVIRONMENT
1. Sodium sulfite + potassium permanganate + potassium hydroxide = .......................
2. Potassium bromide + chlorine + potassium hydroxide = potassium bromate + .......................
3. Manganese(II) sulfate + potassium chlorate + potassium hydroxide = potassium manganate + ...................... .
4. Chromium(III) chloride + bromine + potassium hydroxide = potassium chromate + .......................
5. Manganese(IV) oxide + potassium chlorate + potassium hydroxide = potassium manganate + ...................... .
In an acidic environment
1. Sodium sulfite + potassium permanganate + sulfuric acid = .......................
2. Potassium nitrite + potassium iodide + sulfuric acid = nitric oxide (II) + .......................
3. Potassium permanganate + nitric oxide (II) + sulfuric acid = nitric oxide (IV) + ...................... .
4. Potassium iodide + potassium bromate + hydrochloric acid = .......................
5. Manganese(II) nitrate + lead(IV) oxide + nitric acid = manganese acid +
+ ...................... .
Answers to exercises in task 2
N E U T R A L ENVIRONMENT

Task 3. Using the electron-ion balance method, create ORR equations.
1. Manganese(II) hydroxide + chlorine + potassium hydroxide = manganese(IV) oxide + ...................... .
Electron-ion balance:

2. Manganese(IV) oxide + oxygen + potassium hydroxide = potassium manganate +.......................
3. Iron(II) sulfate + bromine + sulfuric acid = .......................
4. Potassium iodide + iron(III) sulfate = ....................... .
5. Hydrogen bromide + potassium permanganate = ..................................
6. Hydrogen chloride + chromium(VI) oxide = chromium(III) chloride + .......................
7. Ammonia + bromine = .......................
8. Copper(I) oxide + nitric acid = nitric oxide(II) + .......................
9. Potassium sulfide + potassium manganate + water = sulfur + .......................
10. Nitric oxide (IV) + potassium permanganate + water = .......................
11. Potassium iodide + potassium dichromate + sulfuric acid = ..................................
12. Lead(II) sulfide + hydrogen peroxide = ..............................
13. Hypochlorous acid + hydrogen peroxide = hydrochloric acid + .......................
14. Potassium iodide + hydrogen peroxide = ..................................
15. Potassium permanganate + hydrogen peroxide = manganese(IV) oxide + .....................................
16. Potassium iodide + potassium nitrite + acetic acid = nitric oxide (II) + ....................................
17. Potassium permanganate + potassium nitrite + sulfuric acid = ..................................
18. Sulfurous acid + chlorine + water = sulfuric acid + .......................
19. Sulfurous acid + hydrogen sulfide = sulfur + ..............................
water formation. The solution obtained after passing gases through water had an acidic reaction. When this solution was treated with silver nitrate, 14.35 g of a white precipitate formed. Determine the quantitative and qualitative composition of the initial mixture of gases. Solution.
The gas that burns to form water is hydrogen; it is slightly soluble in water. Hydrogen with oxygen and hydrogen with chlorine react explosively in sunlight. It is obvious that there was chlorine in the mixture with hydrogen, because the resulting HC1 is highly soluble in water and gives a white precipitate with AgN03.
Thus, the mixture consists of gases H2 and C1:
1 mole 1 mole
HC1 + AgN03 -» AgCl 4- HN03.
x mol 14.35
When treating 1 mol of HC1, 1 mol of AgCl is formed, and when treating x mol, 14.35 g or 0.1 mol. Mr(AgCl) = 108 + 2 4- 35.5 = 143.5, M(AgCl) = 143.5 g/mol,
v= - = = 0.1 mol,
x = 0.1 mol HC1 was contained in the solution. 1 mol 1 mol 2 mol H2 4- C12 2HC1 x mol y mol 0.1 mol
x = y = 0.05 mol (1.12 l) hydrogen and chlorine reacted to form 0.1 mol
NS1. The mixture contained 1.12 liters of chlorine and 1.12 liters of hydrogen + 1.12 liters (excess) = 2.24 liters.
Example 6. There is a mixture of sodium chloride and sodium iodide in the laboratory. 104.25 g of this mixture was dissolved in water and excess chlorine was passed through the resulting solution, then the solution was evaporated to dryness and the residue was calcined to constant weight at 300 °C.
The dry matter mass turned out to be 58.5 g. Determine the composition of the initial mixture as a percentage.
Mr(NaCl) = 23 + 35.5 = 58.5, M(NaCl) = 58.5 g/mol, Mr(Nal) = 127 + 23 = 150 M(Nal) = 150 g/mol.
In the initial mixture: mass of NaCl - x g, mass of Nal - (104.25 - x) g.
When sodium chloride and iodide are passed through a solution, iodine is displaced by it. When the dry residue was passed through, the iodine evaporated. Thus, only NaCl can be a dry substance.
In the resulting substance: mass of initial NaCl x g, mass of the resulting (58.5-x):
2 150 g 2 58.5 g
2NaI + C12 -> 2NaCl + 12
(104.25 - x) g (58.5 - x) g
2,150 (58.5 - x) = 2,58.5 (104.25-x)
x = - = 29.25 (g),
those. NaCl in the mixture was 29.25 g, and Nal - 104.25 - 29.25 = 75 (g).
Let's find the composition of the mixture (in percent):
w(Nal) = 100% = 71.9%,
©(NaCl) = 100% - 71.9% = 28.1%.
Example 7 68.3 g of a mixture of nitrate, iodide and potassium chloride was dissolved in water and treated with chlorine water. As a result, 25.4 g of iodine was released (the solubility of which in water was neglected). The same solution was treated with silver nitrate. 75.7 g of sediment fell. Determine the composition of the initial mixture.
Chlorine does not interact with potassium nitrate and potassium chloride:
2KI + C12 -» 2KS1 + 12,
2 mol - 332 g 1 mol - 254 g
Mg(K1) = 127 + 39 - 166,
x = = 33.2 g (KI was in the mixture).
v(KI) - - = = 0.2 mol.
1 mole 1 mole
KI + AgN03 = Agl + KN03.
0.2 mol x mol
x = = 0.2 mol.
Mr(Agl) = 108 + 127 = 235,
m(Agl) = Mv = 235 0.2 = 47 (r),
then AgCl will be
75.7 g - 47 g = 28.7 g.
74.5 g 143.5 g
KCl + AgN03 = AgCl + KN03
X = 1 L_ = 14.9 (KCl).
Therefore, the mixture contained: 68.3 - 33.2 - 14.9 = 20.2 g KN03.
Example 8. To neutralize 34.5 g of oleum, 74.5 ml of a 40% solution of potassium hydroxide is consumed. How many moles of sulfur oxide (VI) are there per 1 mole of sulfuric acid?
100% sulfuric acid dissolves sulfur oxide (VI) in any proportion. The composition expressed by the formula H2S04*xS03 is called oleum. Let's calculate how much potassium hydroxide is needed to neutralize H2S04:
1 mole 2 mole
H2S04 + 2KON -> K2S04 + 2Н20 xl mol y mol
y - 2*x1 mole of KOH goes to neutralize S03 in oleum. Let's calculate how much KOH is needed to neutralize 1 mol of S03:
1 mole 2 mole
S03 4- 2KOH -> K2SO4 + H20 x2 mol z mol
z - 2 x2 mol KOH goes to neutralize SOg in oleum. 74.5 ml of 40% KOH solution is used to neutralize oleum, i.e. 42 g or 0.75 mol KOH.
Therefore, 2 xl + 2x 2 = 0.75,
98 xl + 80 x2 = 34.5 g,
xl = 0.25 mol H2S04,
x2 = 0.125 mol S03.
Example 9 There is a mixture of calcium carbonate, zinc sulfide and sodium chloride. If 40 g of this mixture is exposed to excess hydrochloric acid, 6.72 liters of gases will be released, which, upon interaction with excess sulfur (IV) oxide, will release 9.6 g of sediment. Determine the composition of the mixture.
When the mixture was exposed to excess hydrochloric acid, carbon monoxide (IV) and hydrogen sulfide could be released. Only hydrogen sulfide reacts with sulfur (IV) oxide, so its volume can be calculated from the amount of precipitate released:
CaC03 + 2HC1 -> CaC12 + H20 + C02t(l)
100 g - 1 mol 22.4 l - 1 mol
ZnS + 2HC1 -> ZnCl2 + H2St (2)
97 g - 1 mol 22.4 l - 1 mol
44.8 l - 2 mol 3 mol
2H2S + S02 -» 3S + 2H20 (3)
xl l 9.6 g (0.3 mol)
xl = 4.48 l (0.2 mol) H2S; from equations (2 - 3) it is clear that ZnS was 0.2 mol (19.4 g):
2H2S + S02 -> 3S + 2H20.
It is obvious that carbon monoxide (IV) in the mixture was:
6.72 l - 4.48 l = 2.24 l (C02).
Solution:
2Cl2 + 2H2O = 4HCl + O2
mp-pa = m(H2O) + m(Cl2) − m(O2) ;
Δm = m(Cl2) − m(O2) ;
Let us take n(Cl2) to be X, then n(O2) = 0.5x;
Let's create an algebraic equation based on the above equality and find X:
Δm = x M(Cl2) − 0.5x M(O2) = x(71 − 16) = 55x;
x = 0.04 mol;
V(Cl2) = n(Cl2) Vm = 0.004 22.4 = 0.896 l.
Answer: 0.896 l.
10. Calculate the range of permissible values of the volume of chlorine (no.) that is necessary for complete chlorination of 10.0 g of a mixture of iron and copper.
Solution:
Since the condition does not say what the ratio of metals in the mixture is, we can only assume that the range of permissible values for the volume of chlorine in this case will be the range between its volumes required to chlorinate 10 g of each metal separately. And solving the problem comes down to sequentially finding these volumes.
2Fe + 3Cl2 = 2FeCl3
Cu + Cl′2 = CuCl2
n(Cl2) = 1.5n(Fe) = 1.5 10/56 = 0.26 mol;
V(Cl2) = n(Cl2) Vm = 0.26 22.4 = 5.99 ≈ 6 l;
n(Cl′2) = n(Cu) = 10/63.5 = 0.16 mol;
V(Cl′2) = 22.4 · 0.16 = 3.5 l.
Answer: 3.5 ≤ V(Cl2) ≤ 6 l.
11. Calculate the mass of iodine that is formed when treating a mixture of sodium iodide dihydrate, potassium iodide and magnesium iodide with an excess of an acidified solution of potassium permanganate, in which the mass fractions of all salts are equal, and the total amount of all substances is 50.0 mmol.
Solution:
Let's write down the equations of the reactions occurring in the solution and compose the general half-reactions, on the basis of which we will arrange the coefficients:
10NaI 2H2O + 2KMnO4 + 8H2SO4 = 5I2 + 2MnSO4 + 5Na2SO4 + K2SO4 + 28H2O
10KI + 2KMnO4 + 8H2SO4 = 5I2 + 2MnSO4 + 6K2SO4 + 8H2O
5MgI2 + 2KMnO4 + 8H2SO4 = 5I2 + 2MnSO4 + 5MgSO4 + K2SO4 + 8H2O
MnO4¯+ 8H+ + 5ē = Mn2+ + 4H2O 2
2I¯− 2 ē = I2 5
2 MnO4¯+ 16H+ + 10 I¯= 2 Mn2+ + 5I2 + 8H2O
From the equality of the mass fractions of the components of the mixture it follows that their masses are also equal. Mistaking them for X Let's create an algebraic equation based on the equality:
n1 + n2 + n3 = 50.0 mmol
m1/M(NaI 2H2O) + m2/M(KI) + m3/M(MgI2) = 50.0 mmol
m1 = m2 = m3 = x
x/186 + x/166 + x/278 = 50 10-3 mol
m (I2)1 = 5M(I2) m(NaI 2H2O)/10M(NaI 2H2O) = (5 254 3.33)/10 186 = 2.27 g;
m (I2)2 = 5M(I2) m(KI)/10M(KI) = (5 254 3.33)/10 166 = 2.55 g;
m (I2)3 = 5M(I2) m(MgI2)/10M(MgI2) = (5 254 3.33)/10 278 = 3.04 g.
Total: 7.86 g.
Answer: 7.86 g.
12. When passing chlorine through 200 g of a 5.00% solution of hydrogen peroxide, the mass of the solution increased by 3.9 g. Calculate the mass fractions of substances in the resulting solution.
Solution:
Н2О2 + Cl2 = О2 + 2НCl
1. Find the initial amount of H2O2 in solution:
n1(H2O2) = m/M(H2O2) = mP-RA ω/ M(H2O2) = 200 0.05/34 =
2. Let us take the amount of absorbed chlorine in the solution as X, then nO2 = x, and the increase in the mass of the solution is due to the difference in the masses of absorbed chlorine and released oxygen:
m(Cl 2) − m(O 2) = Δ m or x M(Cl 2) − x M(O2) = Δ m;
71x − 32x = 3.9; x = 0.1 mol.
3. Calculate the amount of substances remaining in the solution:
n2(H2O2)OXIDIZED = n(Cl2) = 0.1 mol;
n(H2O2) REMAINING IN SOLUTION = n1 − n2 = 0.294 − 0.1 = 0.194 mol;
n(HCl) = 2n(Cl 2) = 0.2 mol.
4. Find the mass fractions of substances in the resulting solution:
ω(H2O2) = n(H2O2) M(H2O2)/ mP-RA = 0.194 34/203.9 100% = 3.23%;
ω(HCl) = n(HCl) M(HCl)/ mP-RA = 0.2 36.5/203.9 100% = 3.58%.
Answer:ω(H2O2) = 3.23%;
ω(НCl) = 3.58%.
13. Manganese (II) bromide tetrahydrate weighing 4.31 g was dissolved in a sufficient volume of water. Chlorine was passed through the resulting solution until the molar concentrations of both salts were equal. Calculate how much chlorine (no.) was passed through.
Solution:
Mn Br2 4H2O + Cl2 = MnCl2 + Br2 + 4H2O
1. Find the initial amount of manganese (II) bromide tetrahydrate in solution:
n(Mn Br2 · 4H2O)REQ. = m/M = 4.31/287 = 1.5 10−2 mol.
2. Equality of the molar concentrations of both salts will occur when half of the initial amount of Mn Br2 · 4H2O is consumed. That. the amount of chlorine required can be found from the reaction equation:
n(Cl2) = n(MnCl2) = 0.5 n(Mn Br2 · 4H2O) ref. = 7.5·10−3mol.
V(Cl2) = n·Vm = 7.5·10−3·22.4 = 0.168 l.
Answer: 0.168 l.
14. Chlorine was passed through 150 ml of barium bromide solution with a molar salt concentration of 0.05 mol/l until the mass fractions of both salts were equal. Calculate how much chlorine (200C, 95 kPa) was passed through.
Solution:
BaBr2 + Cl2 = BaCl2 + Br2
1. From the equality of the mass fractions of the formed salts, the equality of their masses follows.
m(BaCl2) = m(BaBr2) or n(BaCl2) M(BaCl2) = n′(BaBr2) M(BaBr2).
2. Let us take n(BaCl2) as X mol, and n′(BaBr2), remaining in solution, for SM ·V − x = 0.15·0.05− x = 7.5·10−3− x and compose an algebraic equation:
208x = (7.5 10−3− x) 297;
2.2275 = 297x +208x;
3. Find the amount of chlorine and its volume:
n(Cl2) = n(BaCl2) = 0.0044 mol;
V(Cl2) = nRT/P = (0.0044 8.314 293)/95 = 0.113 l.
Answer: 113 ml.
15. A mixture of potassium bromide and fluoride with a total mass of 100 g was dissolved in water; excess chlorine was passed through the resulting solution. The mass of the residue after evaporation and calcination is 80.0 g. Calculate the mass fractions of substances in the resulting mixture.
Solution:
1. After calcination of the reaction products, the residue consists of potassium fluoride and chloride:
2КBr + Cl2 = 2КCl + Br2
2. Let us take the amounts of KF and KBr as X And at accordingly, then
n(KCl) = n(KBr) = y mol.
Let's create a system of equations based on equalities:
m(KF) + m(KBr) = 100
m(KF) + m(KCl) = 80
n(KF) M(KF) + n(KBr) M(KBr) = 100
n(KF) M(KF) + n(KCl) M(KCl) = 80
58x + 119y = 100 58x = 100 – 119y
58 x + 74.5y = 80 100 – 119y + 74.5y = 80
44.5y = 20; y = 0.45; x = 0.8.
3. Let’s find the masses of substances in the residue and their mass fractions:
m(KF) = 58·0.8 = 46.5 g.
m(KCl) = 74.5 0.45 = 33.5 g.
ω(KF) = 46.5/80·100% = 58.1%;
ω(KCl) = 33.5/80·100% = 41.9%.
Answer:ω(KF) = 58.1%;
ω(КCl) = 41.9%.
16. A mixture of sodium bromide and iodide was treated with excess chlorine water, the resulting solution was evaporated and calcined. The mass of the dry residue turned out to be 2.363 times less than the mass of the original mixture. How many times will the mass of the precipitate obtained after treating the same mixture with an excess of silver nitrate be greater than the mass of the original mixture?
Solution:
2NaBr + HClO +HCl = 2NaCl + Br2 + H2O
2NaI + HClO +HCl = 2NaCl + I2 + H2O
1. Let us take the mass of the initial mixture as 100 g, and the amounts of the salts NaBr and NaI that form it as X And at respectively. Then, based on the ratio (m(NaBr) + m(NaI))/ m(NaCl) = 2.363, we create a system of equations:
103x + 150y = 100
2.363·58.5(x+y) = 100
x = 0.54 mol; y = 0.18 mol.
2. Let's write down the second group of reactions:
NaBr + AgNO3 = AgBr↓ + NaNO3
NaI + AgNO3 = AgI↓ + NaNO3
Then, to determine the ratio of the masses of the formed precipitate and the initial mixture of substances (taken as 100 g), it remains to find the quantities and masses of AgBr and AgI, which are equal to n(NaBr) and n(NaI), respectively, i.e. 0.18 and 0 .54 mol.
3. Find the mass ratio:
(m(AgBr) + m(AgI))/(m(NaBr) + m(NaI)) =
(M(AgBr) x + M(AgI) y)/100 =
(188 0.18 + 235 0.54)/100 =
(126,9 + 34,67)/100 = 1,62.
Answer: 1.62 times.
17. A mixture of magnesium iodide and zinc iodide was treated with excess bromine water, the resulting solution was evaporated and calcined at 200 - 3000C. The mass of the dry residue turned out to be 1.445 times less than the mass of the original mixture. How many times will the mass of the precipitate obtained after treating the same mixture with excess sodium carbonate be less than the mass of the original mixture?
Solution:
1. Let us write down both groups of reactions, denoting the masses of the initial mixture of substances and the resulting products as m1, m2, m3.
(MgI2 + ZnI2)+ 2Br2 = (MgBr2 + ZnBr2)+ 2I2
(MgI2 + ZnI2)+ 2 Na2CO3 = (MgCO3 + ZnCO3)↓ + 4NaI
m1/ m2 = 1.445; m1/ m3 = ?
2. Let us take the amount of salts in the initial mixture as X(MgI2) and at(ZnI2), then the amounts of products of all reactions can be expressed as
n(MgI2) = n(MgBr2) = n(MgCO3) = x mol;
n(ZnI2) = n(ZnBr2) = n(ZnCO3) = y mol.








 About the company Foreign language courses at Moscow State University
About the company Foreign language courses at Moscow State University Which city and why became the main one in Ancient Mesopotamia?
Which city and why became the main one in Ancient Mesopotamia? Why Bukhsoft Online is better than a regular accounting program!
Why Bukhsoft Online is better than a regular accounting program! Which year is a leap year and how to calculate it
Which year is a leap year and how to calculate it